Immunomonitor
Research project

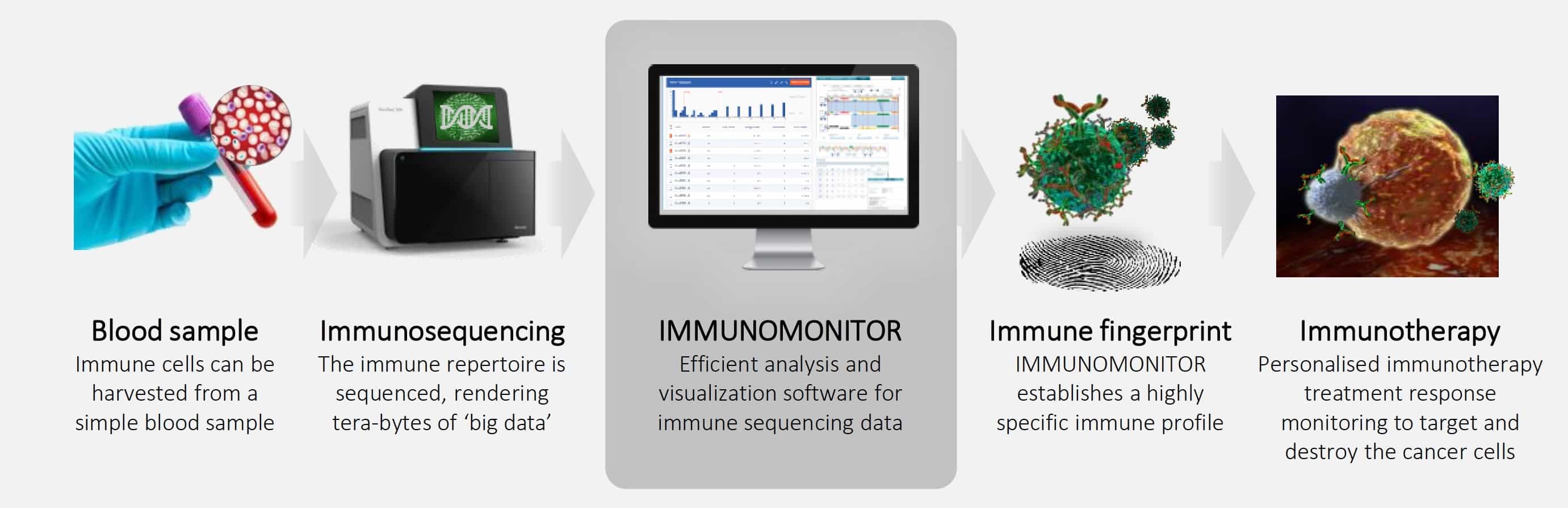
Treatment response monitoring for cancer immunotherapies using immune repertoire analysis
Learn more
Biomax joins four partners in the IMMUNOMONITOR cancer research project, which aims to validate a data analysis and visualization software solution for treatment response monitoring of cancer immunotherapies based on over-time immune repertoire sequencing analysis.
Biomax will drive the integration of longitudinal NGS data from multi-center cancer vaccine studies and accompanying clinical data capturing systems into the BioXM platform. The study data will be harmonized and used to develop novel NGS analysis algorithms.
More details
Back to top
